Ferroelectric Ceramic
A ferroelectric material is one whose equilbrium state has a bulk electric dipole moment. This occurs in solid ceramics when ground state crystal structure involves spatial separation of ionic charges, and the unit cell lacks a center of symmetry. Nanoscale alignment of the microscopic electric dipole moments is responsible for bulk ferroelectric behavior. Typically, the magnitude of the dipole polarization and its orientation can be controlled by application of modest external electric fields. This leads to piezoelectric behavior: generation of electric fields in response to external stress and conversely, crystal deformation in response to external electric fields. The unique properties of ferroelectric materials give them many important applications as computer memories, switches and actuators, and ultrasonic transducers. A good introduction to this subject can be found in a monograph, "Ferroelectric Crystals" by F. Jona and G. Shirane [Dover, New York, 1993] and a review article, "Relaxor Ferroelectrics: an Overview", by L.E. Cross, [Ferroelectrics 151, 305, (1994)].
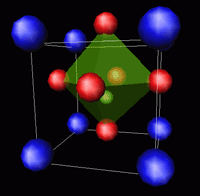 Many ferroelectric ceramic materials are based on the perovskite structure, ABO3, in which positively charged A cations (blue) and negatively charged O anions (red) occupy body-centered cubic lattice sites, while smaller B cations (green) occupy an interpenetrating lattice in which they are surrounded by six oxygens in an octahedral arrangement.
Many ferroelectric ceramic materials are based on the perovskite structure, ABO3, in which positively charged A cations (blue) and negatively charged O anions (red) occupy body-centered cubic lattice sites, while smaller B cations (green) occupy an interpenetrating lattice in which they are surrounded by six oxygens in an octahedral arrangement.
This image was obtained from http://www.ill.fr.dif/3D-crystals/perovskites.html, a site where many useful discussions of perovskite structure may be found. Ionic charge, size mismatch, and covelent tendency combine to produce distortions from cubic symmetry, without which piezoelectric and ferroelectric properties would not exist. The perovskite structure is very robust, and supports multiple substitutions at both A and B sites. Solid solutions can be prepared over a wide range of composition without phase separation, thereby permitting bulk properties to be "tuned" by careful control of chemical composition and post-synthesis processing. Understanding and control of the distorted structures at the unit cell level are clearly crucial for intelligent design of new materials.
Relaxor ferroelectrics are characterized by a broad, frequency dependent maximum in dielectric loss near the paraelectric-ferroelectric phase transition. The study of these materials received tremendous impetus in the early 1990's with the discovery that single crystals of lead magnesium niobate/lead zirconate (PMN/PZ) solid solutions and related materials displayed very large, highly anisotropic piezoelectric behavior accompanied by very large remnant polarization [ see T.R. Shrout, et al. , ferroelectric Letters 12, 63, 1990 and S.-E. Park, et. al., J. Mater. Sci., 29, 1284 (1994). Numerous applications of these materials have been developed; a web search in March, 2005 for "PMN Applications" gave over 33,000 hits! Despite a decade of intensive research, the microscopic origins of this behavior are still controversial and not fully understood. There appears to be general agreement that relaxor behavior arises from polar nanodomains with a high degree of local dipole alignment, but the relation of these domains to underlying, partially ordered ionic arrangements remains elusive. "Positional disorder" in relaxor ferroelectrics refers to the fact that ionic displacements from ideal, cubic perovskite lattice sites may differ in more or less random fashion from unit cell to unit cell. "Chemical disorder" is a term used by physicists to indicate random identity of chemical elements at A and/or B sites in mixed perovskites and solid solutions.
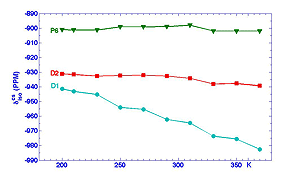
Magnetic resonance of quadrupolar nuclei is an especially powerful tool for examining local structure in disordered ferroelcectric ceramics. NMR signals can be obtained from polycrystalline powders as well as single crystals, and most of the relevant A and B site ions have NMR active isotopes which are easy to detect at natural abundance. With the advent of high field spectrometers ( > 14T), fast magic angle spinning (> 30 KHz), and two dimensional multiple quantum techniques, it is possible to obtain accurate values of site-specific NMR chemical shifts and quadrupole coupling parameters. This is important, because the NMR parameters can be identified with particular local ionic configurations on the basis of model compound studies, ab initio calculations, and semi-empirical relations. For example, our studies of 95Nb resonance in the lead magnesium niobate/lead scandium niobate system provided the first unambiguous, quantitative experimental evidence in favor of Davies' random site model of chemical (dis-)order.
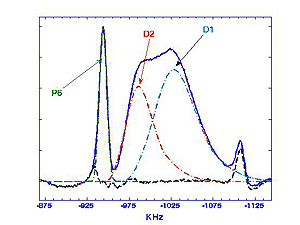
More recently, analysis of temperature dependent MAS and 3QMAS spectra taken in our laboratory at 17.6T reveal interesting differences in the behavior of Nb5+ cations that have different configurations of next nearest B-site neighbors (nBn). The MAS spectrum of PMN, Figure 1, can be decomposed into three ovelapping peaks. The narrow peak, P6, is assigned to the configuration with six Mg2+ nBn cations. This peak is narrow because the electric field gradient in this symmetric configuration is relatively small. The "distribution" peak D1 consists of unresolved signals from configurations containing at least one Nb5+ cation and zero or one Mg2+ cations ; D2 includes configurations with 2-5 Mg2+ cations. Note that the simulated line shapes for D1 and D2 (dashed lines) are asymmetric. This happens because there is a Gaussian distribution of quadrupole coupling constants, CQ, for each peak, and the peak frequency depends on the square of CQ.